Home » Neuroscience (Page 3)
Category Archives: Neuroscience
#SfN14 highlights: Multimodal Investigation of Large-Scale Brain Dynamics: Combining fMRI and Intracranial EEG
007.Minisymposium. Multimodal Investigation of Large-Scale Brain Dynamics: Combining fMRI and Intracranial EEG – Biyu He.
Saturday, Nov 15, 2014, 1:30-4 PM
I made it to DC almost in time to attend all the talks of the mini-symposium on the multimodal investigation of large-scale brain dynamics through functional MRI and intracranial EEG (nice job, Megabus). Altogether, I found that the talks were of a very high technical level, and a good grasp of the analysis techniques generally employed in those studies (especially in intracranial EEG) definitely helped me follow the finer points made by the speakers.
Here are a few highlights from the session. My apologies to the speakers whose talks I did not cover. Note that any inaccuracy or outright misunderstanding in what follows is my responsibility alone!
7.03. Large-scale patterns of cortical rhythmic suppression in human cerebral cortex – Christopher Honey
In his talk, Dr. Honey, from the University of Toronto, presented results on the relationship between cortical low-frequency rhythms (think theta, alpha and low beta, or between 4 and 30 Hz) and high-gamma power (HGP, approximately 70 to 180 Hz), a non-rhythmic portion of the intracranial EEG’s power spectrum that is a good proxy for local neuronal firing. The data came from intracranial electrodes implanted in patients with epilepsy who were going to have surgery in an attempt to remove the focus of their seizures. When the patients were performing an audiovisual task (watching a movie), Honey observed an inverse relationship between the power of the low-frequency alpha rhythm and that of the high-gamma band in the occipital cortex. Similarly, he found anti-correlation between the perirolandic beta rhythm (an oscillation that is most intense when the patients were idling, as opposed to moving their hands for instance) and HGP. At a more global level, he noted that the frequency that was most strongly anti-correlated with HGP varied across cortical areas (it was mostly theta in the temporal lobe and a mixture of theta and low beta in the prefrontal cortex). Crucially, however, that frequency was also the same one that dominated the power spectrum of spontaneous oscillations. Honey found a similar relationship between the low frequencies that displayed the highest modulation of HGP (so-called cross-frequency phase-power coupling) and spontaneous oscillations. He therefore put forward the idea that these low-frequency cortical rhythms may provide pulsed inhibition of local cortical activity (suppressive rhythms). Intriguingly, Honey also observed maximal anti-correlations between the power of low-frequency rhythms and the BOLD signal (collected before the patients were implanted with electrodes), which serves as a reminder that HGP and BOLD probably reflect partly the same underlying neuronal mechanisms.
Note that a question that remains open is why are different cortical areas oscillating at different baseline frequencies. I wonder whether it reflect some basic periodic or rhythmic property of e.g. visual inputs to the occipital cortex or motor outputs from the motor cortex…
7.04. Cognitive electrophysiology of the human medial parietal cortex: Local and network dynamics – B. Foster
Dr. Foster, from Stanford University, focused on the parietal cortex, parts of which have often been observed to be more active when subjects were apparently “not doing much” in the functional MRI scanner (hence they were dubbed part of the so-called default mode network, DMN). It is also known that the parietal cortex–especially the medial aspect, the posterior cingulate cortex (PCC) and retrosplenial cortex (RSC)–also activate during the retrieval of autobiographic memories. Dr. Foster and colleagues therefore designed an intracranial EEG task that would allow disentangling the engagement of those cortical areas during autobiographical retrieval tasks as opposed to more general, non self-centered memory retrieval or arithmetic. He found that, indeed, the PCC and RSC were selectively activated by self-episodic and self-semantic retrieval tasks. Interestingly, the angular gyrus also showed activation, and the two areas displayed correlated activity across single trials, validating the idea that they are part of a coherent functional network during those tasks.
7.05. Cross-frequency coupling in the cortical columnar microcircuit – A. Maier
Dr. Maier, from Vanderbilt University (Nashville, TN), explored cross-frequency phase-power coupling using intracortical recordings across the layers of the cerebral cortex in monkeys (so-called laminar recordings). He found that HGP in the supra- and infra-granular layers of the cerebral cortex are coupled to the phase of alpha oscillations, whereas HGP in the granular layer (that receives feed-forward input from the thalamus) did not display such a relationship. Dr. Maier used current source density (CSD) analysis to determine that the infra-granular layers were likely responsible for the drops in firing rate throughout the thickness of the cortical column. Therefore, he hypothesized, layer 6 neurons might be responsible for transiently shutting down the propagation of feed-forward activity through the cortical column micro-circuit, allowing instead feed-back inputs (that mostly reach the supra- and infra-granular layers) to exert their influence.
7.07. Multimodal imaging of spatio-temporal dynamics in language processing – T. Thesen
Dr. Thesen, from New York University, focuses on the processing of both written and spoken language using a combination of functional MRI, magnetoencephalography (MEG, a technique that records very similar signals to EEG) and intracranial EEG in epilepsy patients. The first experiment that he presented was concerned with written language. Using strings of pseudo-letters vs. pseudo-words made up entirely of consonants vs. actual words, he showed that there was a spatial gradient in the complexity of responses, ranging from specific to real letters vs. pseudo-letters in more posterior parts of the occipito-temporal cortex to specific to real words vs. pseudo-words in more anterior parts of that visual processing stream. Interestingly, Dr. Thesen’s use of electrophysiological techniques also revealed a temporal gradient, in that letter-specific responses started earlier after the appearance of the stimuli than word-specific responses.
In a second study, Dr. Thesen took advantage of the well-characterized McGurk effect (an audiovisual illusion caused by mismatched auditory and visual speech syllables–check out this Youtube video for an example!). When contrasting the response of the brain to audiovisual syllables to the combination of audio-only or video-only stimuli, he found that multisensory effects appeared very early in the auditory cortex (40 ms after sound onset), then a bit later in the left superior temporal sulcus (80 ms), and later again in the left inferior frontal gyrus (120 ms). The very early multisensory effects in the auditory cortex point to a direct, feed-forward effect of visual stimuli through the visual cortex to the early auditory areas. Interestingly, Dr. Thesen’s data even point towards the role of pre-stimulus activity in the visual cortex in the multisensory effects observed in the auditory cortex.
Altogether, a very interesting symposium with talks of a very high technical level that presented for the most part unpublished results. The attendance looked great, with several people sitting on the floor behind the rows of chairs!
Dr. Thesen’s results in particular were very interesting to me, as my own research tackles very similar subjects using similar methods. Check out my poster, Tuesday afternoon, where I’ll be happy to tell you more about this fascinating subject! 623.06/DD4. Phase tracking of visual speech in the human auditory cortex revealed by intracranial EEG.
What I’ll blog about at #SfN14
I’m happy to announce that I’ll be one of the Society for Neuroscience’s official bloggers during the 2014 Annual Meeting, starting this Saturday November 15 in Washington DC. What does it mean? Essentially, that the SfN links to my blog (I will also try and cross-post on the SfN’s own platform, NeurOnLine–see here, for instance). Note that I’m also an editor for the PLOS Neuroscience Community, and we’ve put together a group of bloggers and tweeters who will provide collaborative coverage of the conference. So, given the many sources of information about the meeting that are available to you, why should you read this blog at all? Here, I’ll try to tell you what I intend to write about, so that you can make up your mind (and perhaps also learn about that awesome poster session that you somehow missed when preparing your itinerary!).
I like studying brains in their working environment, the body, hence I’ll focus on in vivo studies. I will report mostly on human neuroscience, with some work in other mammals thrown in (I worked with mice and rats during my PhD). My interests range from sensory perception through multisensory integration to cognition. As someone with dual training in neuroscience and neurology, I’m always interested in work on diseases or in patients–particularly those suffering from epilepsy. My method of predilection is electrophysiology, and especially intracranial EEG in humans, therefore I’ll be reporting on various studies that use that approach. Also, video games and music are important to me, so you can expect that I’ll be keeping an eye on studies about either (or both: if your poster title is “Experienced players of Rock Star have better multisensory discrimination abilities: an EEG study”, give me a call!). Finally, I spend most of my time on the poster floors, because I appreciate the interaction with the authors that posters allow, and that you don’t get at a talk.
What follows is a tentative list of what I would like to attend:
Sunday 1-3 PM: The Neuroscience of Gaming (WCC 201). I’ll post something about this one for sure.
Sunday 1-5 PM: Functional mechanisms of attention 1 (posters RR16-RR40).
On Sunday afternoon, I also warmly recommend the Symposium on studying human cognition with intracranial EEG and electrical brain stimulation, but since I already saw half of the talks at Human Brain Mapping 2014 this summer, I’ll pass this time.
Monday 8-12 AM: Oscillations: EEG (posters C37-C59). I’m especially happy to announce my colleague David Groppe’s poster, Electrocorticographic oscillatory connectivity predicts low frequency resting state functional magnetic resonance imaging connectivity. The man has a blog, too, and an excellent one at that! His presentation time is 10 AM.
Monday 8-12 AM: Multisensory and Temporal Factors in Cross-Modal Processing (posters DD8-DD25).
Also on Monday morning at 8 AM, I’ll try to visit poster M6, An EEG methodology to localize the irritative cortices in a preclinical model of focal epilepsy, by the group of J. Riera from Miami, and poster VV17, Elucidating brain network dynamics with resting-state fMRI and electrocorticography, from J. Parvizi’s team in Stanford. That’s going to mean a lot of walking, but hey, SfN is work!
Monday 3:15-4:25 PM: Cellular and Molecular Mechanisms of Explicit Learning in the Hippocampus – Roger A. Nicoll (WCC Hall D). The large SfN lectures are often extremely good–if you attended last year’s meeting in San Diego, you might remember the stellar presentation given by Doris Tsao. I’m very much looking forward to hearing Dr. Nicoll review long-term potentiation (LTP), our best candidate for the neural substrates of memory.
Tuesday 8-12 AM: Auditory Processing: Temporal, Frequency, and Spectral Processing-Perception (posters FF2-FF12).
Tuesday 1-5 PM: Multisensory: Cross-Modal Processing in Humans, Audio-Visual (posters CC35-DD11). I’ll be presenting my own poster, Phase tracking of visual speech in the human auditory cortex revealed by intracranial EEG. Yay! My presentation time is 2 PM.
Another poster on which I worked will be presented by my PI, Ashesh Mehta, Tuesday at 3 PM (SS18): Failure of the default mode network to deactivate precedes attentional lapses: An intracranial EEG study.
Also on Tuesday afternoon are interesting poster sessions on Human Studies of Epilepsy (posters L10-O1) and Auditory Processing: Human Studies of Perception, Cognition, and Action (posters CC7-CC34). I probably won’t have the time to check those out in detail, but once you are done visiting my poster, you might want to give them a look!
Wednesday 1-4 PM: Predictive Coding: Human Cognition (WCC 152A).
Two of my colleagues, Ido Davidesco and Corey Keller, will present their work, A causal role of the fusiform face area in face perception, on Wednesday at 4 PM (poster II19) (you’ll have to postpone that early flight back home, I’m afraid).
Now, all work and no play makes Jack a dull boy: you can expect the odd post about the commercial exhibition, the bus ride to DC, the social events, the best burger/ramen/pizza within 5 blocks of the convention center…
Announcing The Neuroscience of Gaming at SfN 2014
Here I briefly introduce one of the sessions I will blog about at SfN 2014, together with a short interview of Dr. Adam Gazzaley, one of the speakers at the session. The definitive version of this post was originally published on November 11 2014 on the PLOS Neuroscience Community website, where I serve as an editor.
ME08. The Neuroscience of Gaming. Social Issues Roundtable
Sunday, Nov 16, 2014, 1:00 PM — 3:00 PM. WCC 201
Featured speaker: Adam Gazzaley
One of the sessions that I’m most looking forward to at the SfN’s 2014 annual meeting is the Roundtable on the Neuroscience of Gaming. Why? Well, I started playing video games since I was in primary school, and they have been part of my life ever since. The Roundtable will bring together four speakers with varied backgrounds and expertise:
- Daniel Greenberg is the president of Media Rez, a software and game development studio based in Washington DC whose goal is to develop games that support behavior change in health and learning.
- Adam Gazzaley directs a cognitive neuroscience lab at the University of California in San Francisco that focuses, among others, on developing custom-made video games to improve cognitive functions.
- Mark Griffiths, from Nottingham Trent University, in the UK, specializes in behavioral addictions such as gambling and video gaming addictions.
- Martha Farah, who heads a cognitive neuroscience lab the University of Pennsylvania, will focus on the ethical and social aspects of using neuroscience in games.
I’m particularly interested in hearing about Dr. Gazzaley’s work. I’ve been thinking for a while that video games, in addition to being a great form of entertainment (and a cultural artefact!), represent fantastic tools to probe the function of the brain. Think about it: you are being exposed to complex, multisensory stimuli (obviously visual and auditory, but also tactile through vibrating controllers for instance), to which you have to respond by specific motor commands in a precise temporal window. The whole thing is orders of magnitude more engrossing than a typical psychophysical experiment, yet the basic principles remain. If you are able to intervene in the design of the game to have the player perform a task in which you’re interested, retrieve from the system when game events are happening, and synchronize that with measurements of brain activity, you can use video games as a very powerful scientific tool.
Well, Dr. Gazzaley and his team are doing precisely that — and more, since (among others) they are also adding non-invasive brain stimulation to their armamentarium. If you would like to know more about Dr. Gazzaley’s previous and current work, look to his study on how training on a custom-designed video game improved cognitive abilities in older adults, which was published by Nature last year (it was also highlighted on the cover, and it’s a very good one, too). More recently, Dr. Gazzaley was interviewed in a New York Times Magazine article on the controversy surrounding the “brain training” industry. He is also among the signatories of a recent consensus paper from the scientific community on the same issue. We can expect to hear more about this fascinating subject during the Roundtable.
Five Questions to: Adam Gazzaley
What, in your view, is the one most exciting finding that neuroscience has produced?
Evidence describing the anatomy and physiology of neuroplasticity.
If you could use a magic wand to improve one aspect of how research is conducted, what would you do?
The convergence of different perspectives and methodologies on the same issue would be more common.
What aspect of your research work do you prefer?
Working as a team with a group of intelligent and inspired people.
Is your career similar to what you had in mind as an undergraduate?
Similar, but more involved with health that I ever imagined.
What advice would you give an undergraduate neuroscience major (or recent graduate) about how best to advance in the field?
Search what you love to do, and don’t be afraid to think different.
I would like to thank Dr. Gazzaley for taking the time to answer my questions. I hope that I have convinced you to attend the Social Issues Roundtable on the neuroscience of gaming — but in case you missed the live session, I’ll blog about it, so keep watching this space!
Mapping human brains in the operating room
The definitive version of this post was originally published on October 29 2014 on the PLOS Neuroscience Community website, where I serve as an editor.
Modern brain mapping calls on a number of non-invasive techniques, such as functional MRI or EEG, to investigate which parts of the brain participate in which neurological and cognitive functions. But, in the unfortunate event that you would suffer from a brain disorder requiring surgery, your doctor would not design her operative strategy on an fMRI scan–at least, not on fMRI alone: she would most likely resort to direct electrical stimulation of your cerebral cortex to map out the areas that are absolutely necessary for you to speak or move your hand. In a study recently published in Brain, Tate and colleagues report the results of just such a procedure in a large cohort of patients at the University Hospital of Montpellier, France. Their collected experience will prove extremely useful for clinicians and scientists involved in clinical brain mapping. In addition, their findings challenge the role of Broca’s area, traditionally thought to be crucial for speech output.
How the brain was mapped
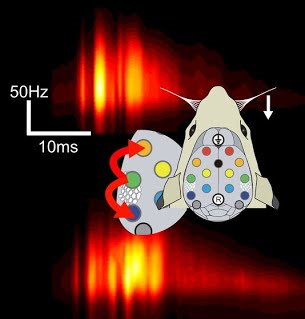
In the study, the authors performed brain mapping during surgery in 165 patients suffering from low-grade gliomas, slow-growing brain tumors. The procedure is called awake craniotomy because direct cortical stimulation mapping requires the patient to speak and move; thus, the surgery is performed under local as opposed to general anesthesia. The neurosurgeon applied an electrode to points on the patient’s cerebral cortex and intermittently delivered stimulation while the patient was asked to count or name pictures. Electrical stimulation of a patch of cortex at frequencies of around 50 Hz for a couple of seconds transiently impaired the function of that area, offering a glimpse of what would befall the patient if that area were removed or damaged. A neuropsychologist or a speech therapist present in the operating room graded the patient’s spoken output, differentiating anomia (the inability to name objects despite being able to speak), dysarthria (improperly articulated speech), paraphasias (the replacement of a word or part of a word by another one, related either semantically or phonologically), or speech arrest (the incapacity to speak at all). Sites where speech was altered by stimulation (“hits” in the jargon) were then mapped onto a standard atlas of the brain’s surface to allow comparisons between patients. Sites that induced motor or somatosensory responses were also mapped, which confirmed the method’s accuracy in that the vast majority of those hits were found in the precentral and postcentral gyri, respectively.
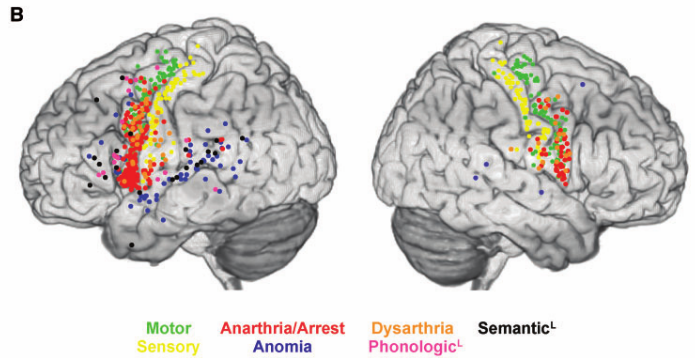
A summary of Dr. Dufffau’s team’s brain mapping results. Figure copyright of the article authors, used with their permission.
The authors used cluster analysis to summarize the anatomical locations where each type of language disturbance was most commonly observed. The detailed results of the study, beautifully presented on maps of the brain surface with colored dots indicating the effect of cortical stimulation on each area, will likely be referred to often by professionals who perform similar procedures as well as neuroscientists interested in the anatomical correlates of cognitive functions.
Revisiting Broca’s
Tate and colleagues found that dysarthria most often resulted from stimulation of the lateral precentral and postcentral gyri of both hemispheres, implicating the somatosensory cortex in the network necessary for proper articulation. Crucially, they observed that speech arrest, often considered a good marker of Broca’s area, in fact most often resulted from perturbation of the ventral premotor cortex, irrespective of whether the left or right hemisphere was stimulated. By contrast, stimulation of Broca’s area itself (the opercularis and triangularis parts of the left inferior frontal gyrus, according to the anatomical definition) very rarely caused speech arrest; patients more often produced semantic and phonological paraphasias. These findings attribute a role to Broca’s area in higher-order aspects of speech production rather than as a motor or premotor output region. Anomia and paraphasias were generally found in the superior temporal and inferior frontal regions of the left hemisphere.
Many strengths and a few weaknesses
This study is exceptional in several aspects: the high number of patients (single-center functional MRI studies of language very rarely enroll so many participants!), the rigorous protocol for testing speech production, and the clarity of the graphic representation of the results. There are a couple of shortcomings to the study as well: the anatomical location of sites that failed to disrupt speech was not recorded in detail, so that the probabilistic aspect of the cortical maps presented here only applies at the level of entire gyri. Also, by mapping stimulation sites to a template rather than to the patients’ own cortical anatomy, the authors “discarded” information that would have been helpful to investigate the variability of anatomical-functional relationships in the human cortex. Nevertheless, these minor drawbacks do not diminish the major significance of the present study in any respect.
Future perspectives
Is there a chance that functional brain mapping with non-invasive methods will one day allow physicians to dispense with direct cortical stimulation? Not according to corresponding author Professor Hugues Duffau: “Non-invasive brain mapping using functional neuroimaging will never be able to localize language functions as accurately as invasive stimulation, because fMRI or DTI [diffusion tensor imaging, a technique that uses MRI to image fiber tracts in the brain’s white matter] are only able to provide indirect information on brain processes.”
Another frequently highlighted difference between fMRI and direct stimulation studies of cognitive functions is that the former reveals all the cerebral areas that participate in a given task, whereas the latter focuses on those that are absolutely necessary in order to accomplish it, and is thus the preferred approach in the specific situation of neurosurgery.
Direct cortical stimulation is not limited to the exploration of speech production: Prof. Duffau and his team have also probed the anatomical correlates of semantic and syntactic processing, visual-verbal congruence judgments, spatial awareness, mentalistic inferences (the attribution of mental states to others), and the anatomical correlates of consciousness. More generally, direct electrical stimulation of the brain offers unique insights into the localization of neurological and cognitive functions in humans, and future studies will likely continue to enrich our understanding of the human brain’s anatomical and functional organization.
References
Tate, M., Herbet, G., Moritz-Gasser, S., Tate, J., & Duffau, H. (2014). Probabilistic map of critical functional regions of the human cerebral cortex: Broca’s area revisited Brain, 137 (10), 2773-2782 DOI: 10.1093/brain/awu168
Publication bias in psychology
The definitive version of this post was originally published on September 8, 2014 on the PLOS Neuroscience Community website, where I serve as an editor.
An article published recently in PLOS ONE, Publication Bias in Psychology: A Diagnosis Based on the Correlation between Effect Size and Sample Size, by Kühberger, Fritz and Scherndl, generated healthy discussion on social media.
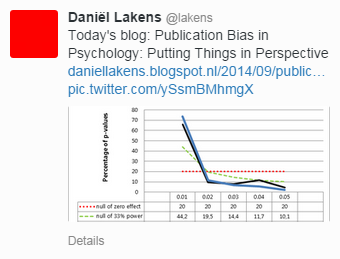
Kühberger and colleagues, from the University of Salzburg in Austria, discuss the relationship between effect size and sample size. These two quantities, they argue, should normally be unrelated. However, the authors found a clear correlation between the two, mostly driven by studies with sample sizes below 100. They also looked at the distribution of p values and found that barely-significant values were much more frequently reported than values that just failed to reach the conventional threshold for statistical significance. Both findings, the authors argue, reflect publication bias in psychology.
“There seems to be agreement that small sample studies are at the center of the problems around publication bias. If so, neuroscience (especially traditional imaging studies with their sample sizes of about 20) could be especially affected”, Kühberger said.
”Publication bias is a problem in science (Everyone, 1950-2014).”
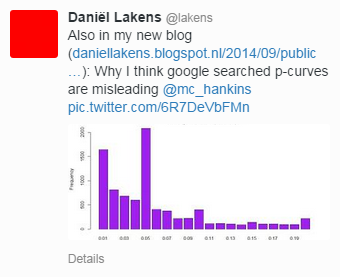
In an informative blog post, Daniël Lakens (from the Eindhoven Institute of Technology, The Netherlands) takes the discussion further, especially with respect to p curves (plots that illustrate the distribution of p values across studies). There is a risk of misinterpreting these p curves as a sign of scientific malconduct (“p-hacking”); what they reflect instead is precisely publication bias, Lakens indicates. He concludes that “[Kühberger et al’s] article is an excellent reminder of how publication bias is a huge waste of resources, and biases the effect size estimates based on the published literature.”
References
Kühberger, A., Fritz, A., & Scherndl, T. (2014). Publication Bias in Psychology: A Diagnosis Based on the Correlation between Effect Size and Sample Size PLoS ONE, 9 (9) DOI: 10.1371/journal.pone.0105825
Spatial-temporal resolution plots for neuroscience methods
You must have seen these plots before, where the temporal resolution of various methods of probing brain function is plotted along one axis and their spatial resolution on the other. Spatial resolution is often approximated in terms of units of the nervous system (from dendritic spines through neurons and cortical columns all the way to lobes and hemispheres). Similarly, temporal resolution is indicated with easy-to-understand labels, from milliseconds to hours and beyond.
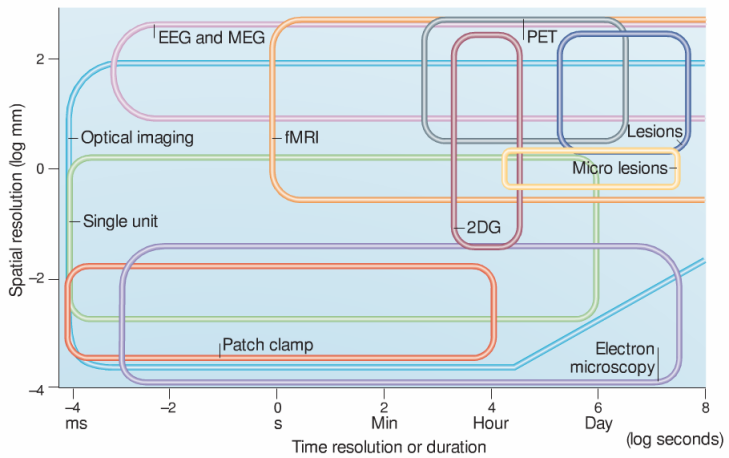
One of the most complete spatial-temporal resolution plots for neuroscience methods. Notice how the spatial resolution of fMRI stretches down to the millimeter, whereas the temporal resolution of EEG reaches the millisecond. The spatial and temporal sampling (or coverage) of each method is depicted by the height and the width of each box, respectively. From Grinvald and Hildesheim, 2004. Reproduced with permission from Nature Publishing Group.
Thus, functional MRI, which can resolve brain activity down to the millimeter and to the second, occupies a different position on the plot than EEG, whose temporal resolution is much more accurate (within the millisecond) but whose spatial resolution is more on the scale of centimeters. The techniques that boast the highest resolution in both space and time are generally more invasive: intracerebral micro-electrode arrays are a prime example.
These plots also present a crucial piece of information when assessing methods: the extent to which they can sample the brain’s function. This is generally done by drawing an area for each technique that depicts the span that it covers in both dimensions. This notion of sampling or coverage is critical given our current understanding of major cerebral functions being subtended by large-scale networks of neurons that link remote areas into cohesive units. Thus, micro-electrodes can resolve individual neurons, but it is practically impossible to record from more than a few small patches of brain at a time.
The same sampling problem applies to time: micro-electrodes can record high-quality brain signals for days to weeks to a few months, but scarring around the implanted material tends to alter the properties of the signals in the very long term. By contrast, there is no a priori technical hurdle to placing the same subject in the fMRI scanner every day for their entire life.
Using a third dimension
In addition to resolution and coverage, some of the plots go further and use a third dimension (pseudo-3D isometric plots or colors) to represent another feature of the method. For instance, Walsh and Cowey illustrate whether a given method allows interfering with the function of the brain; examples include microstimulation and transcranial magnetic stimulation (TMS).
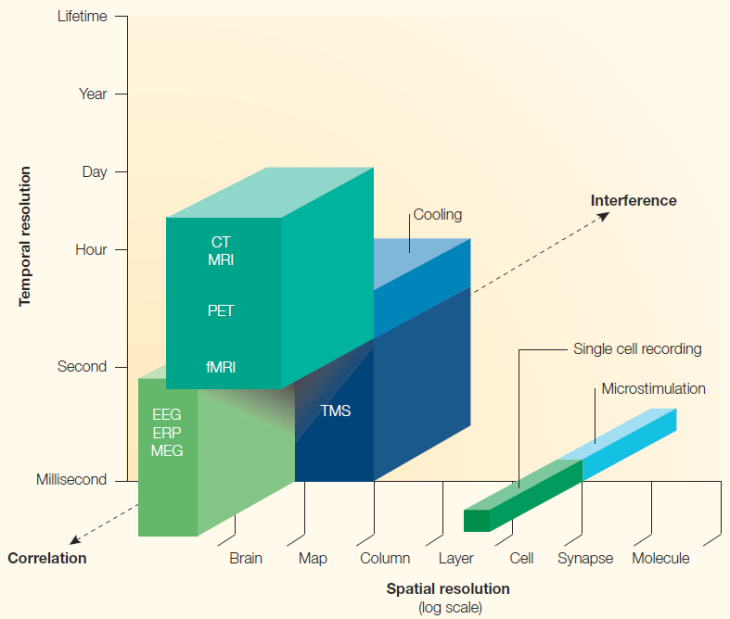
In this plot, a third dimension is used to illustrate whether a given method allows interfering with brain function, instead of recording its correlates. From Walsh and Cowey, 2000. Reproduced with permission from Nature Publishing Group.
In another example, Devor and colleagues nicely use color to show how different optical imaging methods are able to penetrate through the thickness of the brain.
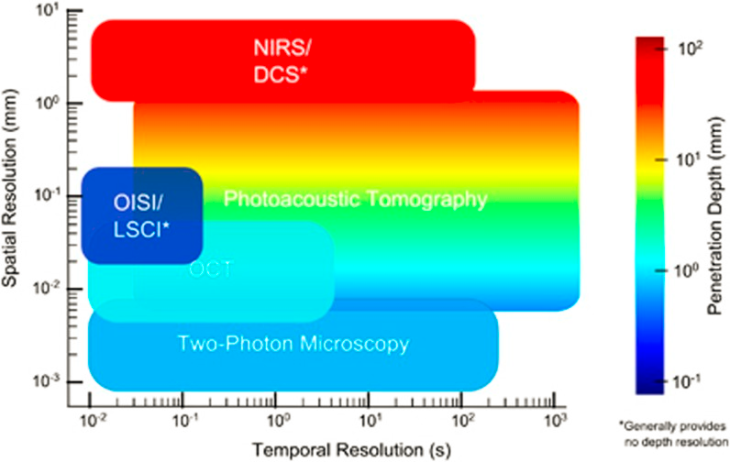
Here, color provides an added dimension, encoding the depth to which each technique can penetrate the depth of the brain tissue. NIRS: near-infrared spectroscopy; DCS: diffuse correlation spectroscopy; OISI: optical intrinsic signal imaging; LSCI: laser speckle contrast imaging; OCT: optical coherence tomography. From Devor et al., 2012. Reproduced with permission from Nature Publishing Group.
My final example, taken from an article by Mehta and Parasumaran, uses the third dimension to represent how much a given method forces the subject (human, in this case) to remain immobile. Obviously, fMRI and MEG, where the sensors are fixed to heavy machinery, and not attached to the subject’s head as are EEG electrodes of NIRS sensors, ideally require perfect immobility. This is likely to become a fundamental aspect of neuroscience methods, as neuroscience moves further towards more naturalistic, ecologically valid experimental paradigms.
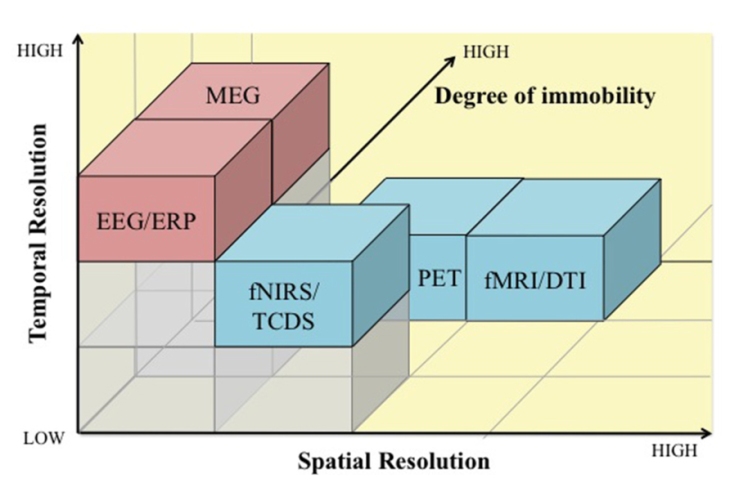
Here, the third dimension represents how much each technique interferes with the subject’s ability to move as data are being acquired. From Mehta and Parasuraman, 2013. Published under a Creative Commons Attribution license (CC BY).
To sum up, any given approach to investigating brain function has a number of dimensions that affect its performance and its adequacy to address a particular question. Spatial-temporal resolution plots for neuroscience methods are a good way of representing this complex dimensionality and a nice example of how one well-designed image can transmit a wealth of information.
References
Grinvald, A., & Hildesheim, R. (2004). VSDI: a new era in functional imaging of cortical dynamics Nature Reviews Neuroscience, 5 (11), 874-885 DOI: 10.1038/nrn1536
Walsh, V., & Cowey, A. (2000). Transcranial magnetic stimulation and cognitive neuroscience Nature Reviews Neuroscience, 1 (1), 73-80 DOI: 10.1038/35036239
Devor, A., Sakadžić, S., Srinivasan, V., Yaseen, M., Nizar, K., Saisan, P., Tian, P., Dale, A., Vinogradov, S., Franceschini, M., & Boas, D. (2012). Frontiers in optical imaging of cerebral blood flow and metabolism Journal of Cerebral Blood Flow & Metabolism, 32 (7), 1259-1276 DOI: 10.1038/jcbfm.2011.195
Mehta, R., & Parasuraman, R. (2013). Neuroergonomics: a review of applications to physical and cognitive work Frontiers in Human Neuroscience, 7 DOI: 10.3389/fnhum.2013.00889
“Hello, hive!” Brain scientists report first direct brain-to-brain communication in humans
The definitive version of this post was originally published on September 8, 2014 on the PLOS Neuroscience Community website, where I serve as an editor.
Scientists have, for the first time, assisted two human beings in communicating through mental processes across two continents. They reported the results of their experiment in PLOS ONE on August 19, 2014. But if this exciting first step has your mind racing with ideas of telepathy, freely reading the streams of thoughts of your fellow human beings, hold on: for now, the current brain-to-brain experiment amounts more to a painstaking conversation in Morse code. It also raises crucial ethical issues with respect to the privacy of our mental processes and our sense of agency.
In the experiment, the researchers, based in Spain and France, used a brain-computer interface to allow a human ‘emitter’ to encode a message, and a computer-brain interface so that ‘receivers’ could decode it. The emitter, located in India, generated a message (a binary form of the word ‘hello’) by thinking of either of two things: moving their feet or their hands. The researchers used EEG to differentiate the electrical activity generated by the brain in these two situations, allowing them to translate the emitter’s thoughts into a binary code (0 when the emitter thought of moving the feet, 1 for the hands) that was sent to France over the internet. There, the receivers sat underneath a transcranial magnetic stimulation (TMS) system that transmitted the binary code to them through noninvasive brain stimulation.
TMS delivers brief but intense magnetic fields to the brain through the skull, transiently modifying its activity. When TMS is applied to the motor cortex, for instance, muscles in the arm twitch; when applied to the visual cortex in the occipital lobe, TMS causes the subject to perceive a flash of light called phosphene. The research team here chose the latter: the binary code sent from India was thus translated into TMS pulses that either induced phosphenes or not in the receivers. The receivers then simply announced whether or not they had perceived a phosphene, deciphering the binary word one bit at a time.
This experiment represents the first time that people exchanged information by consciously controlling and monitoring their own mental processes, without any active motion or actual sensory perception. Limitations on the technology that is currently available to stimulate the brain noninvasively made the process slow and not entirely error-proof: it took about 30 seconds for a single bit of information to be transmitted, and the error rate was 5 to 10%. Additionally, the message was written in a binary code not directly understandable by humans. Nevertheless, this experiment is an exciting first step towards the possibility of someday accessing the mental processes of another human being—and also, perhaps, of controlling them.
As such, this experiment raises perhaps more than its fair share of critical ethical questions. As mentioned above, noninvasive stimulation of the motor cortex triggers involuntary movements. Dr. Giulio Ruffini, the corresponding author, explains that his team chose not to stimulate the motor cortex because the receivers would then have been able to detect the communication via their peripheral nervous system (they would have sensed their own involuntary motion by proprioception), and the information exchange would therefore not have been a purely ‘brain-to-brain’ one. Nevertheless, the current research introduces the possibility that one human’s thoughts could control another human’s actions remotely, as if it were a puppet or a robot. Of course, current brain stimulation technologies are far too crude yet, but it is arguably only a question of time and of refining our understanding of motor planning and execution before we can consider such an experiment.
More generally, the idea of remotely accessing and influencing another person’s private thoughts is as chilling as it is exciting, and the current research affords a peek into this Pandora’s box. As Dr. Ruffini puts it, “technology empowers us for good and ill.” Neuroscientists will have to maintain the highest ethical standards in pursuing this line of research. They will also need the help of experts in disciplines such as computer security to ensure that the information exchanged remains protected from undesirable attention or interference. But the most profound impact of the current study might lie elsewhere: Dr. Ruffini reveals that “the original project behind this research was called HIVE”, in reference to “super-organisms in which individuals act as a collective.” Neuroscience research, more than any other, has the potential to fundamentally alter the organization of our social structures.
References
Grau, C., Ginhoux, R., Riera, A., Nguyen, T., Chauvat, H., Berg, M., Amengual, J., Pascual-Leone, A., & Ruffini, G. (2014). Conscious Brain-to-Brain Communication in Humans Using Non-Invasive Technologies PLoS ONE, 9 (8) DOI: 10.1371/journal.pone.0105225
I’ve become a PLOS Neuroscience community site editor!
I’m happy to announce that I have recently become an editor for the PLOS Neuroscience community website! That site functions as a venue for informal discussion of neuroscience research, with an emphasis on the open access to scientific findings provided by PLOS. Neuroscientists, your contributions are welcome!
My first post, which I’ll re-post here 24 hours after it first appears at PLOSNeuro, talks about the recent report in PLOS ONE of direct brain-to-brain communication between human subjects. I’m especially happy about the title of the post, “Hello, hive!” Let me (and the other members of the neuroscience community) know what you think by your comments!